NGAL test rapid test assay
| Min. Order: | 1000 Piece/Pieces |
|---|---|
| Trade Term: | FOB,CFR,CIF,DAT,FAS,DDP,DAP,CIP,CPT,FCA,EXW |
| Payment Terms: | Paypal, L/C, D/P, D/A, T/T, WU, Money Gram |
| Supply Ability: | 1,000,000.00/Month |
| Place of Origin: | Jiangsu |
Company Profile
| Location: | Nanjing, Jiangsu, China (Mainland) |
|---|---|
| Business Type: | Manufacturer |
Product Detail
| Model No.: | NRM-FI-1000 |
|---|---|
| Means of Transport: | Ocean, Air, Land |
| Brand Name: | NORMAN |
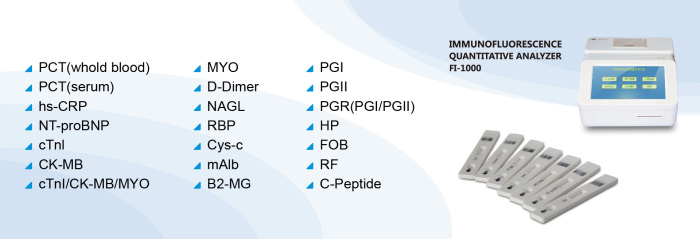
| Methodology: | Fluorescence Immunoassay |
| Model No.: | NRM-FI-1000 |
| Production Capacity: | 1,000,000.00/Month |
| Packing: | In carton |
| Brand Name: | Norman |
| Test time: | 15mins |
| Place of Origin: | Jiangsu, China (Mainland) |
| Manufacturer: | Independent R & D |
| Service: | International after-sales |
| Delivery Detail: | depend on quantity |
| Production Capacity: | 1,000,000.00/Month |
| Packing: | IN CARTON |
| Delivery Date: | TWO WEEKS AFTER PAYMENT |
Product Description
Quantitative IVD Rapid Test Kits
For NGAL
Methodology
Fluorescence immunoassay
Product Name
General Name:NGAL Test Kit(Immunofluorescence)
NGAL
The optimal marker for early diagnosis of acute kidney injury(AKI)
Independent risk index to evaluate the progression of chronic kidney disease(CKD)
NGAL Urine Specs
Methodology | Fluorescence Immunoassay |
Specimen | urine |
Measuring range | 5-1500ng/ml |
Cut-off value | 5ng/ml |
Reaction time | 15 minutes |
Shelf life | 12months |
Package
Test card: Aluminum foil pouch 1pc/bag, desiccant
Package size: 25pcs/box
Intended Use
The test kit is primarily for the in vitro quantitative determination of NGAL content in
human urine. The molecular weight of NGAL is approximately 25kDa, covalent bindings
in neutrophil gelatinase. NGAL is a kind of growth factor which is mainly involved in
occurrence and growth of early renal epithelium. It is low in epithelium of healthy tissue
such as kidney, lung, stomach and colon. In the process of ischemic and toxicity of renal
injury, NGAL in renal tubular epithelial cells will be significantly increased and in the first
two hours, urine NGAL levels will be increased significantly. Therefore, the NGAL is a
sensitive marker of early acute kidney injury. NGAL is also a kind of new potential maker
reflecting chronic kidney damage. It is a powerful and independent risk indicators which
can exactly reflect the level of kidney damage in chronic kidney disease patients.
Principle of the Procedure
The reagent is based on immunefluorescence sandwich assay to detect NGAL
concentrations quantitatively. Using the pipette provided, drop diluted sample into the
sample well. During testing, specimen reacts with the particles coated with anti- NGAL
monoclonal antibody I which has conjugated with fluorescent latex. The mixture migrates
upward on the nitrocellulose membrane by capillary action to react with anti-NGAL
monoclonal antibody II on the test line. The intensity of fluorescent antibody signal reflects
the amount of captured NGAL. Immunefluorescence quantitative analyzer produced by
our company can detect the concentration of NGAL in the sample.
Clinical significance
1.Optimal indicator for early diagnosis of acute kidney injury.
2.Real-time risk stratification of acute kidney injury.
3.Treatment and detection of acute kidney injury and prognosis evaluation.
Applicable Instruments
1.The reagent box must be stored from 4 to 30℃, and the period of validity is 12 months.
2.The reagent strip must be used within 1 hour once its foil pouch has been opened.
3.Do not use the strip beyond the expiration date printed on the outside of the box.
Specimen Collection and Preparation
1.The blood plasma specimen is suggested to use 1: 9 Na-Citrate blood collection
tube for collecting and the specimen is suggested to be tested within 4hours.
2.The specimens must be returned to room temperature before testing.
Test Method
Immunefluorescence techniques
Instruction for use
1.Please read the manual and instrument operation carefully before using.
2.Check whether the equipment can operate regularly, and prepare other related materials.
3.Allow the test device, buffer and specimen balanced to room temperature
(15-30℃) prior to testing. Minimizing exposure to damp air.
Test Procedure
1.Open the Immune-fluorescence quantitative analyzer.
2.Check whether the batch number of IC card and reagent box is consistent, and
then insert IC card into the instrument to get the curve we wanted.
3.Remove the test device from the protective pouch and place it on a flat surface.
Draw in 30μl of blood plasma sample to the centrifugal tube, and dilute with 90μl
of diluents. After gently mix the sample, add 90ul of diluted sample into the sample
well. Place the test strip at room temperature for 10 minutes.
4.Insert the test card into the hole of immune-fluorescence quantitative analyzer, and
click start, the instrument will scan the test card automatically.
5.Immune-fluorescence quantitative analyzer detects results automatically and
calculates the content of D-Dimer in the sample.
6.Specimens, used strip and transfer pipettes may be potentially infectious. Proper
handling and disposal methods should be established by the laboratory direction
in accordance with local regulations.
Product show


Package & Delivery

Related Products
About us
Nanjing Norman Biological Technology Co., Ltd is dedicated to R&D and manufacturing
of automated chemiluminescence system. Founded in 2008, Norman biological has been
upholding the idea that R&D shapes future ,and concentration determines success. Ever
since the beginning, Norman has been focusing on developing and manufacturing
chemiluminescence instruments and reagents.
Norman's manufacture center is located in the Yuhua District and owns an over 2,000m2
GMP-approved clean workshop. The R&D base, which is over 2,000m2 , is located in state-
level new Jiangbei district. Now there are over 100 R&D engineers, 40% of which hold a
PhD or master's degree.



Agent Wanted
If you are interested in working with us, please feel free to contact.

OEM will be highly welcome!
Welcome to our website!