

Chemiluminescence immunoassay PCT procalcitonin reagents
| Min. Order: | 1000 Piece/Pieces |
|---|---|
| Trade Term: | FOB,CFR,CIF,DAT,FAS,DDP,DAP,CIP,CPT,FCA,EXW |
| Payment Terms: | Paypal, L/C, D/P, D/A, T/T, WU, Money Gram |
| Supply Ability: | 1,000,000.00/Month |
| Place of Origin: | Jiangsu |
Company Profile
| Location: | Nanjing, Jiangsu, China (Mainland) |
|---|---|
| Business Type: | Manufacturer |
Product Detail
| Model No.: | NRM-411 |
|---|---|
| Means of Transport: | Ocean, Air |
| Brand Name: | NORMAN |
| Brand Name:: | Norman |
| Place of Origin: | Jiangsu, china (Mainland) |
| Methodology: | chemiluminescence |
| Delivery Detail: | depend on quantity |
| Manufacturer: | Independent R & D |
| Delivery Date: | Two weeks after payment |
| Packing: | In carton |
| Model No.: | NORMAN 411 |
| Production Capacity: | 1,000,000.00/Month |
| Packing: | IN CARTON |
| Delivery Date: | TWO WEEKS AFTER PAYMENT |
Product Description
Chemiluminescence immunoassay PCT procalcitonin reagents
Product show
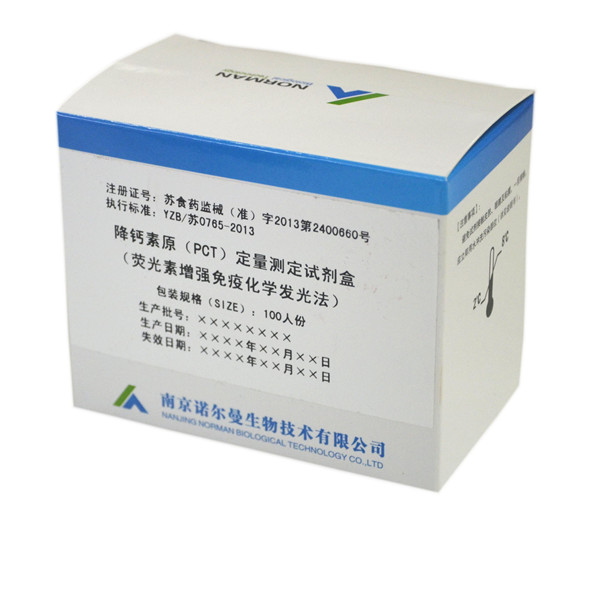 PCT
PCT
Registration No: Jiangsu food and drug administration 2014-2400660
Initiatedchemiluminescenceperipheralwholoe blood PCT testing, especially recommended for children's hospitals and pediatric outpatient /emergency test. Highaccuracy, recommended to purchase aftercomparison test with Roche.
Clinical significance
PCT is a powerful auxiliary tool for infectious diseases diagnosis, stratification, therapy and
prognostic evaluations well as reasonable use of antibiotics. Doctors should raise attention
degree towards PCT test. Start diagnosis and treatment monitoring from patient
suspected of infectious diseases enter hospital, lay the foundation for the subsequent
treatment.
PCT Theclinic and emergency application expert consensus - Chinese Journal of Emergency
Medicine (No. 9,Volume 21,September 2012)
CLIA kits description
Chemiluminescence Immunoassay (CLIA) detection using Microplate luminometers
provides a sensitive, high throughput, and economical alternative to conventional
colorimetric methodologies, such as Enzyme-linked immunosorbent assays (ELISA).
ELISA employs a label enzyme and a colorimetric substrate to produce an amplified signal
for antigen, haptens or antibody quantitation. This technique has been well established and
considered as the technology of choice for a wide variety of applications in diagnostics,
research, food testing, process quality assurance and quality control, and environmental
testing. The most commonly used ELISA is based on colorimetric reactions of chromogenic
substrates, (such as TMB) and label enzymes.
Recently, a chemiluminescent immunoassay has been shown to be more sensitive than the
conventional colorimetric method(s), and does not require long incubations or the addition
of stopping reagents, as is the case in some colorimetric assays. Among various enzyme
assays that employ light-emitting reactions, one of the most successful assays is the
enhanced chemiluminescent immunoassay involving a horseradish peroxidase (HRP)
labeled antibody or antigen and a mixture of chemiluminescent substrate, hydrogen
peroxide, and enhancers.
Performance analysis
Correlation analysis of testing result between Norman whole body PCT and Roche PCT
Applicable department
Pediatrics, ICU, emergency, respiratory, surgical, hospital ward, internal medicine, oncology, hematology
Correlation analysis of testing result between Norman whole body PCT and Biomerieux PCT
Product advantage
1.Advanced technology: initiate peripheral whole blood chemiluminescence PCT test
2.High sensitivity: 0.02ng/ml
3.High accuracy:99% correlation with Roche ELECSYS BRAHMS PCT
4.Wide linear range: 0.02 ∽ 100ng/ml
5.Good repeatability: CV≤5%
Company profile
Nanjing Norman Biological Technology Co., Ltd is dedicated to R&D and manufacturing
of automated chemiluminescence system. Founded in 2008, Norman biological has been
upholding the idea that R&D shapes future ,and concentration determines success. Ever
since the beginning,Norman has been focusing on developing and manufacturing
chemiluminescence and immunofluorescence reagents.

Norman's manufacture center is located in the Yuhua District and owns an over 2,000m2
GMP-approved clean workshop. The R&D base, which is over 2,000m2 , is located in state-
level new Jiangbei district. Now there are over 100 R&D engineers, 40% of which hold a
PhD or master's degree.
Powered by advanced technology and excellent talents in the IVD field, Norman has been
consistently improving its innovation platform , and increasing R&D investment. Self-
innovation, combined with long-term strategic cooperation with universities and research
institutes and with outsourced technologies, ensures consistent improvement on product
quality. Norman's R&D field has covered instruments, reagents, and raw materials, and has
been entrusted by the Nanjing government to build a R&D center specialized in biological
chemistry and immunity diagnosis. Up to now, Norman has acquired over 20 patents.
Being an expert in automated chemiluminescence analysis , Norman owns independent
and completed intellectual property rights, and its products provide top-notch sensitivity,
precision and accuracy . Thanks to the self-developed antigens and self-manufactured
antibodies, Norman's products features minimized intra-and inter- batch difference.
After 8 years development, Norman is now on the fast track. An over 30,000m2 global
R&D center is in construction, and will hold more than 1,000 R&D engineers in the future.
R&D shapes future, and concentration determines success. Driven by the commitment to
"provide complete solution to improve human health", Norman will consistently do its
utmost to drive the IVD industry forward and contribute more to human health.
Agent Wanted
If you are interested in working with us, please feel free to contact.

Why us
Manufacturer and Exporter for nearly 10 years with self R&D Research Center;
Professional After-Sale service with On-site support worldwide;
CE/ISO13485 Certifications
OEM and ODM are available !