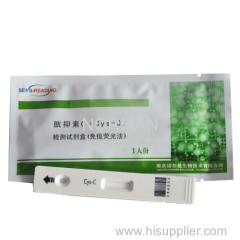

Quantitative In Vitro Diagnostic Rapid Test Kits For Cys-C POCT
| Min. Order: | 1000 Piece/Pieces |
|---|---|
| Trade Term: | FOB,CFR,CIF,DAT,FAS,DDP,DAP,CIP,CPT,FCA,EXW |
| Payment Terms: | Paypal, L/C, D/P, D/A, T/T, WU, Money Gram |
| Supply Ability: | 1,000,000.00/Month |
| Place of Origin: | Jiangsu |
Company Profile
| Location: | Nanjing, Jiangsu, China (Mainland) |
|---|---|
| Business Type: | Manufacturer |
Product Detail
| Model No.: | NRM-FI-1000 |
|---|---|
| Means of Transport: | Ocean, Air, Land |
| Brand Name: | NORMAN |
| Methodology: | Fluorescence Immunoassay |
| Brand Name: | Norman |
| Place of Origin: | Jiangsu, China (Mainland) |
| Test time: | 15mins |
| Packing: | In carton |
| Delivery Date: | Two weeks after payment |
| Manufacturer: | Independent R & D |
| Service: | International after-sales |
| Delivery Detail: | Depend on quantity |
| Type: | NRM FI-1000 |
| Production Capacity: | 1,000,000.00/Month |
| Packing: | IN CARTON |
| Delivery Date: | TWO WEEKS AFTER PAYMENT |
Product Description
Quantitative In Vitro Diagnostic Rapid Test Kits For
Cys-C POCT
Methodology
Fluorescence Immunoassay
Product Name
General Name:Cys-C Test Kit(Immunofluorescence)
Package
Test card: Aluminum foil pouch 1pc/bag, desiccant
Package size: 25pcs/box
Metabolic Characteristics
1.Constant production rate. not affected by age, sex and nutritional status.
2.Freely pass through glomerular filtration, no secretion from tubular.
3.Completely absorbed in proximal tubule.
4.Completely degraded after being absorbed, no longer enter the loop.
Clinical Significance
1.As the indicator of renal injury, Cys-C is better than creatinine, urea nitrogen, etc. 2.Sensitivity of Cys-C in serum detecting diabetic and nephropathy is better than micro proteinuria and relevant indicators.
3.Cys-C can distinguish glomerulus and renal tubular disease, it is the optimal indicator of early diabetic and hypertensive nephropathy.
Test Procedure
1.Open the Immune-fluorescence quantitative analyzer.
2.Check whether the batch number of IC card and reagent box is consistent, and then
insert IC card into the instrument to get the curve we wanted.
3.Remove the test device from the protective pouch and place it on a flat surface. Draw
in 5μl of blood serum sample to the centrifugal tube, and dilute with 400μl of diluents.
After gently mix the sample, add 90μl of diluted blood serum sample into the sample well.
Place the test strip at room temperature for 5 minutes.
4.Insert the test card into the hole of immune-fluorescence quantitative analyzer, and click
start, the instrument will scan the test card automatically.
5.Immune-fluorescence quantitative analyzer detects results automatically and calculates
the content of Cys-C in the sample.
6.Specimens, used strip and transfer pipettes may be potentially infectious. Proper
handling and disposal methods should be established by the laboratory direction in
accordance with local regulations.
Expected values/Reference range
The reference range study was conducted based on Cys-C content of 95% of the distribution
range of statistical analysis in 120 healthy people, the result was as follows:
Normal reference values: 0.57~1.01 mg/L
It is recommended that each laboratory established its own reference range, which may be
unique to the population it serves depending upon geographical, patient, dietary, or
environmental factors.
Advantage
1.High sensitivity:0.2mg/l.
2.High accuracy: 99% correlation with LEAMAN.
3.Wide linear range:0.2-10mg/l.
Applicable departments
ICU, CCU, renal internal medicine, urinary , pediatrics, cardiology, gynecology, oncology, clinical laboratory.
Package & Delivery

Related Products
Inflammation/Infection
PCT(whole blood), PCT (surum), hs-CRP
Cardiovascular
NT-proBNP, D-Dimer
cTnI,CK-MB, MYO
Nephropathy
NAGL, RBP,Cys-c, mAlb, B2-MG
Gastrosis
PGⅠ/ PGⅡ/ PGR/ HP/ FOB
Rheumatism
RF
Diabetes
C-Peptide
About us
Nanjing Norman Biological Technology Co., Ltd is dedicated to R&D and manufacturing of automated chemiluminescence system. Founded in 2008, Norman biological has been upholding the idea that R&D shapes future ,and concentration determines success. Ever since the beginning, Norman has been focusing on developing and manufacturing chemiluminescence instruments and reagents.
Norman's manufacture center is located in the Yuhua District and owns an over 2,000m2 GMP-approved clean workshop. The R&D base, which is over 2,000m2 , is located in state-level new Jiangbei district. Now there are over 100 R&D engineers, 40% of which hold a PhD or master's degree.


Agent Wanted
If you are interested in working with us, please feel free to contact.


