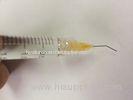
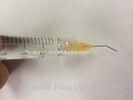
Super Viscosity Medical Sodium Hyaluronate Gel Non Cross Linked / Viscoelastic Devices
| Place of Origin: | Zhejiang |
|---|
Company Profile
| Location: | Weifang, Shandong, China (Mainland) |
|---|---|
| Business Type: | Manufacturer |
| Main Products: | Hyaluronic Acid Dermal Filler, Hyaluronic Acid Fillers, Medical Sodium Hyaluronate Gel |
Product Detail
| Model No.: | HT- Intra Articular Gel |
|---|
Product Description
Super Viscosity Medical Sodium Hyaluronate Gel Non Cross Linked / Viscoelastic Devices

Description
Hi-Tech Medical Sodium Hyaluronate Gel is composed by high purified hyaluronic sodium and physiogical buffer ,and is of colorless and transparent gelatinous solution Hyaluronic sodium can stimulate its own synovial memberance to produce sodium hyaluronate of high molecular weight,which helps alleviate arthralgia,increase the range of mobility,eliminate synovial inflammation and delay the development of the disease.

[Characters]
- High viscosity, large molecular weight
- Outstanding endothelial protection
- Minimizes the risk of developing a shallow of Anterior chamber
[Specifications]
| sodium hyaluronate ophthalmic solution (store at room temperature) |
| PFS - STERILE PRE-FILLED SYRINGE |
| Sodium hyaluronate | PFS |
| 1.0% (10 mg/ml) | 0.5ml, 1.0ml, 1.5ml, 2.0ml /syringe |
| 1.3%, 1.4%, 1.5%, 1.6% (13,14,15,16 mg/ml) | 0.5ml, 1.0ml, 1.5ml, 2.0ml /syringe |
| 1.8% (18 mg/ml) | 0.5ml, 1.0ml, 1.5ml, 2.0ml /syringe |
| 2.0% (20mg/ml) | 0.5ml, 1.0ml, 1.5ml, 2.0ml /syringe |
| 2.3%, 2.4%, 2.5% (23,24,25 mg/ml) | 0.5ml, 1.0ml, 1.5ml, 2.0ml /syringe |
| 3.0% (30 mg/ml) | 0.5ml, 1.0ml, 1.5ml, 2.0ml /syringe |
[Direction & Dosage]
Direction:Administration of intra-articular
Dosage:2ml/time , once a week. 4~5 weeks for a course of treatment.
[Adverse Reactions]
After use, individual patients may appear pain, tetter and pruritus, usually 2-3 days the symptoms may disappear on their own. If the symptoms is persistent, please stop medication and carry out necessary processing.
[Contraindication]
The following situation should prohibit use :
- Hypersensitivity to any component of this product.
- Joint inflammation in acute infections
[Note]
- Only intra-articular and must be intra-articular, if inject into other parts (parenchyma, synovium, ligament) may cause pain or local swelling.
- This product is sterile, non-pyrogenic and strictly aseptic operation. If the packing damaged, please stop using.
- Please extract the liquid loading before injection if there is hydrarthrosis.
[Application]
- Traumatic arthritis
- Osteoarthritis rheumatoid arthritis
- RA(rheumatoid arthritis)
- Scapulohumeral periarthritis
- Tendon repair
- Arthroscopic surgery
- Adhesion of soft tissues
- Joint inspection



