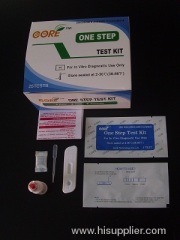
One -Step Strep A Rapid Test
| Min. Order: | 100 Piece/Pieces |
|---|---|
| Trade Term: | EXW |
| Payment Terms: | T/T, WU |
| Supply Ability: | Strep A |
| Place of Origin: | Guangdong |
Company Profile
| Location: | Shenzhen, Guangdong, China (Mainland) |
|---|---|
| Business Type: | Manufacturer |
| Main Products: | In Vitro Diagnostic |
Product Detail
| Model No.: | RH0506I |
|---|---|
| Means of Transport: | Ocean, Air, Land |
| Brand Name: | SAPN |
| Production Capacity: | Strep A |
| Packing: | in house standard |
| Delivery Date: | within 7 working days |
Product Description
A rapid test for the qualitative detection of Strep A antigen in throat swab specimens.For professional in vitro diagnostic useonly.
INTENDED USE
The Strep A Rapid Test Strip (Throat Swab) is a rapid chromatographic immunoassay for the qualitative detection of Strep A antigen
from throat swab specimens to aid in the diagnosis of Group A Streptococcal infection.
SUMMARY
Streptococcus pyogenes is non-motile gram-positive cocci, which contains the Lancefield group A antigen that can cause serious
infections such as pharyngitis, respiratory infection, impetigo,endocarditis, meningitis, puerperal sepsis, and arthritis.1 Left untreated,
these infections can lead to serious complications, including rheumatic fever and peritonsillar abscess.2 Traditional identification
procedures for Group A Streptococci infection involve the isolation and identification of viable organisms using techniques that require
24 to 48 hours or longer.3,4
The Strep A Rapid Test Strip (Throat Swab) is a rapid test to qualitatively detect the presence of Strep A antigen in throat swab
specimens, providing results within 5 minutes. The test utilizesantibodies specific for whole cell Lancefield Group A Streptococcus to
selectively detect Strep A antigen in a throat swab specimen.
DIRECTIONS FOR USE
Allow the test device, specimen, reagents, and/or controls to reach room temperature (15-30°C) prior to testing.
1. Remove the test device from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the test is
performed immediately after opening the foil pouch.
2. Hold the Reagent A bottle vertically and add 4 full drops (approximately 240 uL) of Reagent A to an extraction test tube. Reagent A
is red in color. Hold the Reagent B bottle vertically and add 4 full drops (approximately 160 uL) of Reagent B. Reagent B is colorless.
Mix the solution by gently swirling the extraction test tube. The addition of Reagent B to Reagent A changes the color of the solution
from red to yellow. See illustration 1.
3. Immediately add the throat swab into the extraction test tube of yellow solution. Agitate the swab by rotating it at least 10 times.
Leave the swab in the extraction test tube for 1 minute. Then express the liquid from the swab head by rolling the swab againstthe
inside of the tube and squeezing the tube as the swab is withdrawn. Discard the swab. See illustration 2.
4. With arrows pointing toward the specimen, immerse the test strip vertically into the extracted specimen solution and then start the
timer. If the procedure is followed correctly, the extraction solution should not pass the maximum line (MAX) on the test stripwhen the
strip is immersed. See illustration 3.
5. Leave the strip in the extraction tube and wait for the colored line(s) to appear. Read results at 5 minutes. Do not interpret the
result after 10 minutes.
INTERPRETATION OF RESULTS (Please refer to the illustration above)
POSITIVE:Two lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in
the test line region (T). A positive result indicates that Strep A antigen is detected in the specimen.
NEGATIVE:One colored line appears in the control line region (C). No line appears in the test line region (T). A negative result
indicates that Strep A antigen is not present in the specimen, or is present below the detectable level of the test. The patient's
specimen should be cultured to confirm the absence of Strep A infection. If clinical symptoms are not consistent with results, obtain
another specimen for culture.
INVALID: Control line fails to appear. Insufficient specimen volume orincorrect procedural techniques are the most likely reasons for
control line failure.