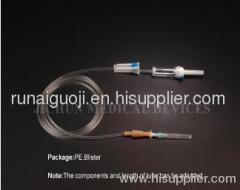
Disposable Infusion Set IV-I DSC-6048
0.09~0.1 USD
| Trade Term: | FOB |
|---|---|
| Payment Terms: | L/C, D/A, T/T |
| Supply Ability: | 0.1billion |
| Place of Origin: | Jiangsu |
Company Profile
| Location: | Changzhou, Jiangsu, China (Mainland) |
|---|---|
| Business Type: | Manufacturer |
| Main Products: | Syring |
Product Detail
| Model No.: | IV-I DSC-6048 |
|---|---|
| Production Capacity: | 0.1billion |
| Packing: | PE, Blister |
| Delivery Date: | 30-60days |
Product Description
Disposable Infusion Set IV-I DSC-6048 is manufactured by Jiangsu Jichun Medical Devices Co., Ltd, who have achieved CE, ISO13485, ISO9001, GMP certificates. 50% products are for Europe, Mideast and Africa markets.
The main accessories of Disposable Infusion Set IV-I DSC-6048 involve Vented spike, Drip Chamber, Fluid Filter, Flow Regulator, Latex Tube, Luer Lock connector.
Protective cap of the hypodermic infusion set for closure piercing device is made of polyethylene with internal thread that prevents the bacteria from coming in, but allows the entrance of ETO gas. Apporoximately 15 drops/ml, 20 drops/ml.
Closure piercing device of disposable sterile infusion set is made of white PVC, with sizes according to ISO 1135-4 standards. Apporoximately 15 drops/ml, 20 drops/ml
Drip chamber is made of soft PVC, sizes according to the ISO 8536-4 standards.
Flow Regulator is made of polyethylene.
Disposable Infusion Set IV-I DSC-6048 hassoft and kink resistant medical grade PVC tubing.
Terminal fitting protective cap (luer slip or Luer-lock adapter upon request) made of PVC or polystyrene, according to the ISO 594/1 and 594/2 standards.
Terminal fitting protective cap made of polyethylene.
Options available:
- With or without air vented spike;
- With or without needle;
- With or without "Y" injection port;
- Luer lock or luer slip connector;
- With or without air vented spike;
- With or without needle;
- With or without "Y" injection port;
- Luer lock or luer slip connector;