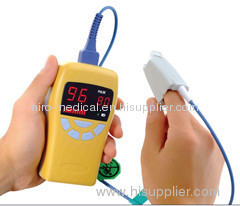
pulse oximeter
| Min. Order: | 1 Set/Sets |
|---|---|
| Payment Terms: | West Union, TT/Paypal |
| Place of Origin: | Hebei |
Company Profile
| Location: | China (Mainland) |
|---|
Product Detail
| Model No.: | CMS50DL |
|---|---|
| Means of Transport: | Fedex, UPS, DHL, EMS, TNT |
| Packing: | Packing Size: 12*7*4 cm |
| Delivery Date: | 3-5 working days after receipt of payment |
Product Description
CONTEC Brand FDA Approved Fingertip Pulse Oximeter Spo2 Monitor CMS 50DL
Product name:CMS 50DL Fingertip Oximeter
Manufacturer: Contec Medical Systems
Model: CMS 50DL Fingertip Oximeter
The Fingertip PulseOximeter is registered on theAustralian Register of Therapeutic Goods (ARTG) with the code 136606,and certified by FDA of United States and CE,TUV of Europe.
CE & FDA Approved Fingertip Pulse Oximeter Spo2 Monitor CMS 50DL 5 colors: blue, yellow, white, black, and pink for choice
CMS-50DL Finger Pulse Oximeter
Technical Parameters
Integrated with SPO2 probe and processing display module
Can be used in: hospital,home,community medical treatment,sports healthcare, etc.
Can measure SPO2 and PR accurately
SPO2 and PR display, bargraph display
Battery voltage low indication
Low power consumption; shut off automatically when no signal
Small volume(57(L) × 33(W) ×32(H) mm)light,convenient to carry
LED display
SPO2: 35-99% Pulse Ratio:30-250BPM
Resolution: 1% for SPO2, 1BPM for pulse ratio
Accuracy: ±2% (7099), unspecified (<70%) for SPO2, ±2BPM or±
2% (select larger) for pulse ratio
Power: 1.5V (AAA size) alkaline batteries × 2
Can work more than 30hours continuously
Interference resistance capacity against ambient light and measurement performance
at low perfusion
Content:
1. Pulse oximeter
2. Carry case
3. Landyard
4. Manual
Wattanty
All items are brand new, with 3 years warranty and whole life repair
About Us
We, Contec are professional medical equipment manufacturer for 17 years in China, we offer patient monitor, fetal doppler, fetal monitor, pulse oximeter, ECG, ECG Holter, EEG, EMG/EP, stethoscope.
Certifications from CONTEC Medical systems:
CE & ISO certification
FDA K Number
CMS50C Pulse Oximeter
Premarket Submission Number(510K): K073454
Listing Number: D045684
CMS50D/L/DL Pulse Oximeter
Premarket Submission Number(510K): K082641
Listing Number: D064765
Sonline A/B,Baby Sound A/B Pocket Fetal Doppler
Premarket Submission Number(510K): K082480
Listing Number: D072247
Premarket Submission Number(510K): K073454
Listing Number: D045684
CMS50D/L/DL Pulse Oximeter
Premarket Submission Number(510K): K082641
Listing Number: D064765
Sonline A/B,Baby Sound A/B Pocket Fetal Doppler
Premarket Submission Number(510K): K082480
Listing Number: D072247
CMS50E\CMS50F\CMS60C\CMS60D Pulse Oximeter
Premarket Submission Number(510K): K090671 Listing Number:D078664,
ECG80A ECG machine
Premarket Submission Number(510K): K090936
Listing Number: D081157
Premarket Submission Number(510K): K090671 Listing Number:D078664,
ECG80A ECG machine
Premarket Submission Number(510K): K090936
Listing Number: D081157
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health:
Phone number: 800.638.2041