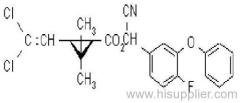
Beta-Cyfluthrin
| Place of Origin: | Shanghai |
|---|
Company Profile
| Location: | China (Mainland) |
|---|---|
| Business Type: | Manufacturer, Trading Company |
Product Detail
| Model No.: | 68359-37-5 |
|---|
Product Description
Common name: beta-cyfluthrin; beta-cyfluthrine; cyfluthrin-beta
IUPAC name: (RS)-cyano-4-fluoro-3-phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name: cyano(4-fluoro-3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
CAS RN: [68359-37-5]
PHYSICAL CHEMISTRY
Composition: Comprises a mixture of four diastereoisomeric pairs of enantiomers:
I (R)-a-cyano-4-fluoro-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate + (S)-a, (1S)-cis-;
II (S)- a, (1R)-cis- + (R)- a, (1S)-cis-;
III (R)- a, (1R)-trans- + (S)- a, (1S)-trans-;
IV (S)- a, (1R)-trans- + (R)- a, (1S)-trans-.
Tech. grade contains: <2% diastereoisomer I, 30-40% diastereoisomer II, <3% diastereoisomer III, and 53-67% diastereoisomer IV. Mol. wt.: 434.3; M.f.: C22H18Cl2FNO3; Form: Colourless crystals; (tech. is a white powder with slight characteristic smell). M.p.: (II) 81 ºC; (IV) 106 ºC; V.p.: (II) 1.4x10-5 mPa; (IV) 8.5x10-5 mPa (both 20 ºC); KOW: (II) logP = 5.94; (IV) logP = 5.91; Henry: (II) 2.9x10-3; (IV) 3.1x10-2 (both in Pa m3 mol-1, 20 °C); S.g./density: 1.34 g/cm3 (22 ºC); Solubility: In water: (II) 2.1, (IV) 1.2 (both in g/l, 20 ºC). In dichloromethane and toluene, both pairs >200 g/l (20 ºC). (II) In hexane 2-5, isopropanol 5-10 (both in g/l, 20 ºC). (IV) in hexane 1-2, isopropanol 2-5 (both in g/l, 20 ºC). Stability: Thermally stable at room temperature. Hydrolysis DT50 (22 ºC) for (II): 117 d (pH 4), 20 d (pH 7), 6 d (pH 9); for (IV): 25 d (pH 4), 11 d (pH 7), 5 d (pH 9).
APPLICATIONS
Mode of action: Non-systemic insecticide with contact and stomach action. Acts on the nervous system, with rapid knockdown and long residual activity.
Uses: Insecticide effective against Lepidoptera, Coleoptera, Hemiptera and Homoptera on cotton, fruit, vegetables, cereals and other crops, at 7.5-20 g/ha. Also against migratory locusts and grasshoppers; and in animal health.
Formulation types: EC; EW; GR; UL; SC.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats c. 500 mg/kg (in polyethyleneglycol), c. 270 mg/kg (xylene), c. 15 mg/kg (cremophor/water); for mice c. 140 mg/kg.
Skin and eye: Acute percutaneous LD50 (24 h) for rats >5000 mg/kg. No irritation to skin and slight primary irritation to the eye (rabbits).
Inhalation: LC50 (4 h) for rats c. 0.1 mg/l (aerosol), 0.53 mg/l (dust).
NOEL: (90 d) for rats 125, dogs 60 mg/kg diet.
ADI: 0.02 mg/kg b.w.
Toxicity class: WHO (a.i.) Ib; EPA (formulation) II
EC hazard T+; R26/28| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for Japanese quail >2000 mg/kg.
Fish: LC50 (96 h) for golden orfe 330.9, rainbow trout 89, bluegill sunfish 28 ng/l.
Daphnia: LC50 (48 h) 0.00029-0.0018 mg/l.
ENVIRONMENTAL FATE
Animals: Beta-cyfluthrin was largely and very quickly eliminated; 98% was eliminated after 48 h via the urine and the faeces.
Plants: Since beta-cyfluthrin is not systemic, it penetrates only slightly into the plant tissues and is hardly translocated into other plant parts. The concentration is very low and can be neglected.
Soil/Environment: Degradation in different soils is rapid. Leaching behaviour can be classified as immobile. The metabolites of beta-cyfluthrin are readily accessible to further microbial degradation to the point of mineralisation to CO2 in the soil.



