Alpha-Cypermethrin
| Place of Origin: | Shanghai |
|---|
Company Profile
| Location: | China (Mainland) |
|---|---|
| Business Type: | Manufacturer, Trading Company |
Product Detail
| Model No.: | 67375-30-8 |
|---|
Product Description
Common name: alpha-cypermethrin; alpha-cyperméthrine
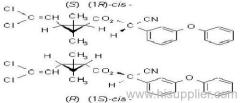
IUPAC name: A racemate comprising (S)--cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and (R)--cyano-3-phenoxybenzyl (1S,3S)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: A racemate comprising (S)--cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and (R)--cyano-3-phenoxybenzyl (1S)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name: [1(S*),3]-(?-cyano(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
Other names: alfoxylate*; alphamethrin* (rejected common name proposals)
CAS RN: [67375-30-8] correct stereochemistry; [52315-07-8] (formerly [86752-99-0], [86753-92-6]) cypermethrin (no stereochemistry stated) were sometimes used in Chemical Abstracts.
PHYSICAL CHEMISTRY
Composition: Tech. grade alpha-cypermethrin is >90% pure m/m, typically >95%. Mol. wt.: 416.3; M.f.: C22H19Cl2NO3; Form: Colourless crystals; (tech. is a white to pale powder, with a weak aromatic odour); M.p.: 78-81 ºC; B.p.: 200 ºC/9.3 Pa; V.p.: 2.3x10-2 mPa (20 ºC); KOW: logP = 6.94 (pH 7); Henry: 6.9x10-2 Pa m3 mol-1 (calc.) S.g./density: 1.28 (22 ºC); Solubility: In water c. 0.01 mg/l (25 ºC). In acetone 620, dichloromethane 550, cyclohexanone 515, ethyl acetate 440, chlorobenzene 420, acetophenone 390, o-xylene 350, hexane 7 (all in g/l, 25 ºC). In maize oil 19-20, ethylene glycol <1 (both in g/kg, 20 ºC). Stability: Very stable in neutral and acidic media. Hydrolysed in strongly alkaline media. Thermally stable up to 220 ºC. Field data indicate that, in practice, it is stable to air and light. F.p. >80 ºC (closed cup).
APPLICATIONS
Mode of action: Non-systemic insecticide with contact and stomach action. Acts on the central and peripheral nervous system in very low doses.
Uses: Control of a wide range of chewing and sucking insects (particularly Lepidoptera, Coleoptera, and Hemiptera) in fruit (including citrus), vegetables, vines, cereals, maize, beet, oilseed rape, potatoes, cotton, rice, soya beans, forestry, and other crops. Control of cockroaches, mosquitoes, flies, and other insect pests in public health; and flies in animal houses. Also used as an animal ectoparasiticide.
Formulation types: EC; SC; TB; UL; WP.
Compatibility: Compatible with most organophosphorus insecticides.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats 79-400 mg/kg (in corn oil, value depending on concentration), 474 mg tech./kg.
Skin and eye: Acute percutaneous LD50 for rats and rabbits >2000 mg tech./kg; minimal irritant to the eyes of rabbits.
Inhalation: LC50 (4 h) for rats 0.32 mg/l air.
NOEL: In 90 d feeding trials, rats receiving 60 mg/kg diet showed no ill-effects.
ADI: 0.02 mg/kg b.w.
Other: Non-mutagenic.
Toxicity class: WHO (a.i.) II; EPA (formulation) II
ECOTOXICOLOGY
Birds: LD50 for quail and mallard ducks >10 000 mg/kg.
Fish: LC50 (96 h) for rainbow trout 0.0028 mg/l; due to rapid loss from water, no toxic effect to fish is observed under field conditions.
Daphnia: LC50 (48 h) 0.1-0.3 g/l.
Bees: Toxic to bees. LD50 (24 h) 0.059 g/bee. No toxic effect under field conditions.
Worms: LD50 (14 d) for earthworms >100 mg/kg artificial soil.
ENVIRONMENTAL FATE
EHC: EHC 142 notes that, although highly toxic to fish, this is not realised under field conditions where rapid loss from water allows recovery of affected populations.
Animals: See cypermethrin.
Soil/Environment: Undergoes degradation in soil, DT50 c. 13 w in loamy soil.




